Phototrophic organisms represent one of the oldest life forms on Earth. During three billion years of evolution, the phototrophs have had to adapt to various environmental conditions including physical (temperature, radiation or pressure) and chemical extremes (desiccation, salinity, pH, oxygen species or redox potential). Among them, high temperatures represent one of the main challenges.
We seek to identify the main molecular factors which are responsible for temperature stability of type 2 photosynthetic reaction centers of anoxygenic phototrophic bacteria, cyanobacteria, green algae and higher plants. We are currently putting in test whether the amino acid mutations which were shown to stabilize cyanobacterial reaction centers will have the same effect also in chloroplasts of algae and higher plants. We are investigating, why are there no truly thermophilic purple bacteria. Do purple bacteria employ similar stabilization mechanism as was shown in cyanobacteria or do they lack this option? We also investigate the role of other membrane components in the temperature stabilization, e.g. the role of small protein subunits in reaction centre stabilization and the role of membrane lipid composition and fatty acid unsaturation.
In cyanobacteria a number of thermophilic strains have been described. Thermosynechococcus elongatus and Thermosynechococcus vulcanus exhibit autotrophic growth optimum at 55 and 57°C respectively. The maximal growth temperature of 74°C found in cyanobacterium Synechococcus cf. lividus is considered to represent the upper limit for oxygenic photosynthesis. The highest growth temperature among anoxygenic phototrophs was found in the green nonsulfur photosynthetic bacterium Chloroflexus aurantiacus which thrives at temperatures of up to 70°C. Interestingly, phototrophic Proteobacteria (purple phototrophic bacteria) seem to lack truly thermophilic species. So far the highest growth optimum of 48 – 50°C was found in the purple sulfur bacterium Thermochromatium tepidum. Only slightly lower growth temperatures, 40 − 48°C, were found in Porphyrobacter tepidarius, a photoheterotrophic bacterium isolated from a brackish thermal spring. A similar growth optimum was also found in the photoheterotrophic bacterium Rubritepida flocculans, however this organism only expressed its photosynthetic apparatus at much lower temperatures.
Light and temperature dependence of photosynthesis in Chlamydomonads isolated from snow
 Snow algae have been reported from polar and mountain regions worldwide. Their survival in the extreme conditions in snow column depends on their ability to adapt their life cycle, structural components of the cells as well as the enzymatic machinery on a molecular level to maintain homeostasis.
Snow algae have been reported from polar and mountain regions worldwide. Their survival in the extreme conditions in snow column depends on their ability to adapt their life cycle, structural components of the cells as well as the enzymatic machinery on a molecular level to maintain homeostasis.We study photosynthesis and its regulation in snow algae belonging to the family Chlamydomonadaceae (Chlorophyta) isolated from red snow above the timberline and also the snow fields that persist long into a late spring in the narrow and deep forested valleys. We focus particularly onto the studies of short living flagellate stages of snow algae that are capable to withstand very high irradiances otherwise lethal for the majority of algae species. At the same time, the snow algae have a potential to effectively utilize very low irradiances as well reflecting their adaptation to diurnal cycle of irradiance in alpine habitats as well as an exponential attenuation of irradiance within the snow column.
press
Past research
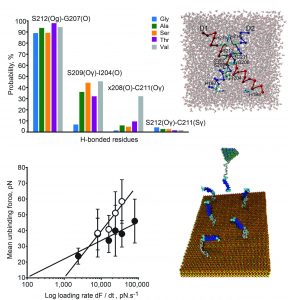
My scientific career has been initiated in the team of Dr. Nedbal at the Institute of Microbiology CAS. Here I have begun to expand my expertise in biochemical and biophysical techniques, particularly optical spectroscopy. I have utilized the gained experience in research projects that included participation in the development of two types of the double-modulated fluorometer (Nedbal et al. 1999, Dijkman et al. 1999) and the kinetic imaging system (Nedbal et al. 2000). I have then successfully exploited the instruments’ potential in establishing a novel approach to study photosystem II function in vivo (Nedbal et al. 1999, Koblížek et al. 2001), in investigation of modulation of photosynthesis on a course of a life cycle in green alga Scenedesmus quadricauda (Kaftan et al. 1999), in a study of effects of iron deficiency on thylakoid membrane structure, composition and function in green alga Dunaliella salina (Varsano el al. 2003) and elucidation of the stress inducing programmed cell death in a filamentous cyanobacterium Calothrix elenkinii (Adamec et al. 2005). I have further gained major experience with atomic force microscopy during my postdoctoral position at the Weizmann Institute in a team of Prof. Reich. I have succeeded in imaging de-enveloped higher plant chloroplasts at nanometer resolution in situ soon (Kaftan et al. 2002) while high resolution live cells’ imaging at higher harmonic frequencies was achieved later in collaboration with a team of Prof. Hinterdorfer at Kepler University in Linz (Dulebo et al. 2009). I have further mastered dynamic force spectroscopy in elucidation of nature and magnitude of forces involved in a molecular recognition on a single-molecule level between proteins of nuclear pore complex (Nevo et al. 2003), oxygen evolving complex of higher plant photosystem II (Kopecký et al. 2012) and between the D1 and D2 membrane proteins of the cyanobacterial photosystem II (Shlyk et al. 2017).
In a past decade I have utilised an interdisciplinary approach that combines biophysical, analytical, molecular biology and computational methods in investigation of molecular mechanisms behind adaptation of photosynthesis to ambient temperature. Together with our partner team led by Prof. Scherz at Weizmann Institute of Science we identified network of hydrogen bonds between locally flexible protein domains that provide stabilization of photosystem II reaction centre core at elevated temperatures (Shlyk-Kerner et al. 2006, Shlyk et al. 2017). We have successfully exploited the potential of the discovered stabilization mechanism in engineering electron and proton transfer in photosystem II enabling a creation of a novel thermotolerant strain of cyanobacterium (Dinamarca et al. 2011, patents Scherz et al. 2008, IL 2007000920; Scherz et al. 2009, EP 2046109; Scherz et al. 2014, US 8629259). The same engineering effort to transform psbA genes encoded in chloroplasts of Chlamydomonas reinhardtii and Nicotinia tabacum however did not result into a thermotolerant phenotypes (Shmidt et al. 2018, Hucková et al. 2018). My recent research primarily focuses onto mechanisms of thermal adaptation of photosynthesis in cryotolerant algae (Kaftan et al. 2011, patent CZ2001-705, Lukeš et al. 2014) in a joint effort with a team of Dr. Nedbalová from Charles University in Prague. The thermotolerance of photosynthesis in photosynthetic bacteria is studied in collaboration with a team of Dr. Koblížek from Institute of Microbiology CAS (Dachev et al., 2017, Piwosz et al. 2018).